
Halogen – One of the 5 non-metals in group 7 of the periodic table.ĭisplacement reaction – A reaction in which a more reactive element takes the place of a less reactive element in a compound.
#HALOGEN REACTIVITY SERIES#
as a Describe the reactivity series competition to see which halogen combines. Halogens become less reactive as you go down group 7 in the periodic table, because the outer electron shell gets further away from the attraction of the nucleus, and so an electron is gained less easily.Ī less reactive halogen will be displaced by a more reactive one from an aqueous solution of its metal halide.Ĭhlorine + potassium bromide → potassium chloride + bromine LeARnIng ouTComes In Topic 12.3 we saw that a more reactive halogen will. Iodine (State of matter at room temperature) Solid (Colour) Grey/ black Why do halogens become less reactive down the group The reactivities of the halogens decrease down the group ( At < I < Br < Cl < F). Halogens' reactivity decreases down the group.

They form hydrogen halides, which dissolve in water, forming acidic solutions.įluorine (State of matter at room temperature) Gas (Colour) YellowĬhlorine (State of matter at room temperature) Gas (Colour) Greenīromine (State of matter at room temperature) Liquid (Colour) Red/ orange Reactivity: Reactivity can be explained as to how easily an element forms compounds with other elements.

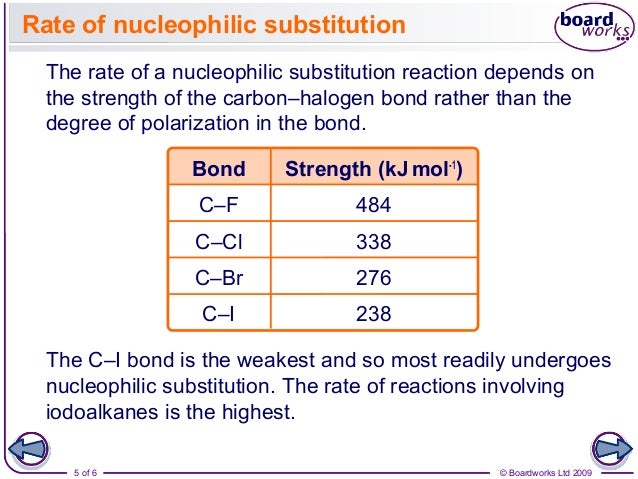
They form molecular compounds with non-metals. They react with metals to form ionic compounds where the halide ion has a charge of -1. They consist of molecules made up of two atoms (diatomic molecules). The halogens have the following properties: 46.Group 7 of the periodic table is home to the Halogens.SERS spectra reveal differences in the reaction product formation for different halogen species, and, on this basis, the possible reaction mechanisms are discussed to approach an understanding of opportunities and limitations in the design of catalytical systems with plasmonic NPs. They are usually found in combination with. In this way, it is revealed how the electronic properties are altered by the adsorption of the ligand molecules, and we conclude that the reaction rates are mainly determined by the plasmonic properties of the AuNPs. The halogens are extremely reactive (especially fluorine), and are not found naturally in their elemental forms. This lack of reactivity is somewhat unlike other nonmetals given the. Complementary information about the electronic properties at the AuNP surface, namely, work-function and valence band states, has been determined by x-ray photoelectron spectroscopy of isolated AuNPs in the gas-phase. The halogen element contains the electron configuration: asthetine, bromine. The halide ions lose electrons and form halogen. Reaction rates are found to be surprisingly similar for different halothiophenols studied here, although the bond dissociation energies of the C–X bonds differ significantly. Displacement reactions of halogens The more reactive halogen atoms oxidise the less reactive halide ions. In the present work, dehalogenation of halogenated thiophenols on the surface of AuNPs has been studied by surface enhanced Raman scattering (SERS) as a function of the photon energy to track the kinetics and identify reaction products. The driving forces of these reactions are typically elevated temperatures, hot charge carriers, or enhanced electric fields. Localized surface plasmon resonances on noble metal nanoparticles (NPs) can efficiently drive reactions of adsorbed ligand molecules and provide versatile opportunities in chemical synthesis.


 0 kommentar(er)
0 kommentar(er)
